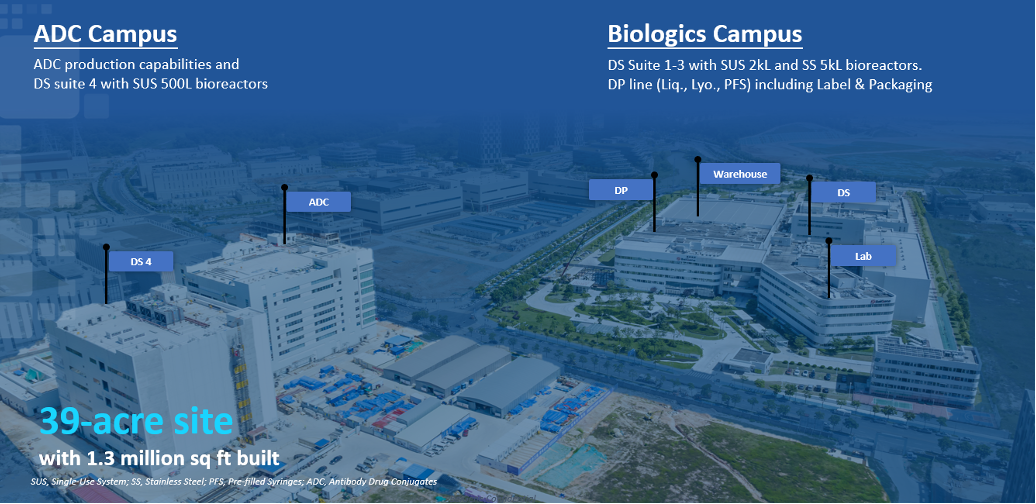
First Biologics Manufacturing Facility
In Guangzhou, we have a 1.3 million sq ft state-of-the-art, commercial-scale facility for the manufacture of large molecule biologics with a production capacity of 65,000 L. We own an adjacent tract of real estate for future expansion to support our growing pipeline of large molecule biologics medicines and drug candidates.

Drug Substance
Manufacturing Capabilities
- Total 65,000L Cell Culture Capacity
- Single-use System (2 x 500L, 12 x 2,000L)
- Stainless System (8 x 5,000L)
- Batch and Fed-batch
Drug Products
End-to-End DP Production Lines
- Liquid Vials (clinical & commercial)
- Lyolized Vials (mAb)
- Pre-filled Syringes (clinical & commercial)
- Label and Packaging

ADCs
Clinical & Commercial Production
- ADC Conjugation room (250L stainless steel tank)
- Partnership with SMOs for Linker & Payload supply
- ADC Purification with Chromatograph & UF/DF
- DP Lyophilized Vials and Label & Packaging

Warehouse
Equipped with Latest Technology
- ASRS (Automatic Search & Retrieval System)
- Storage Conditions: 15 – 25°C, 2-8°C, -40°C, -70°C






